electron arrangement of lithium|Lithium Electronic Configuration : Tagatay Atomic energy shells are subdivided into sub-energy levels. These sub-energy levels are also called orbital. The most probable . Tingnan ang higit pa This is true for other tests, not just OAE tests. Good news: When auditory status is uncertain, it is more likely that we are confusing normal and mild hearing loss. It is much less likely that we are confusing normal hearing with moderate or greater losses.
PH0 · What is the electron configuration of Li+?
PH1 · Lithium Electronic Configuration
PH2 · Lithium Electron Configuration
PH3 · Lithium
PH4 · Li+ Electron Configuration (Lithium Ion)
PH5 · Electronic Configuration of Lithium (Li, Li+ ion)
PH6 · Electron Configuration for Lithium (Li)
PH7 · Chemistry of Lithium (Z=3)
PH8 · 4.7: Arrangements of Electrons
PH9 · 2.7: Electron Configurations
Roulette bets and payouts. In roulette, there are many different types of roulette bets available. Players can bet on a single number or many different groups of numbers. The different types of bets allow players to place bets with a range of winning frequencies, as well as a range of differently sized wins.
electron arrangement of lithium*******The total number of electrons in lithiumis three. These electrons are arranged according to specific rules in different orbitals. The arrangement of electrons in lithium in . Tingnan ang higit pa
Atomic energy shells are subdivided into sub-energy levels. These sub-energy levels are also called orbital. The most probable . Tingnan ang higit pa
Scientist Niels Bohr was the first to give an idea of the atom’s orbit. He provided a model of the atom in 1913. The complete idea of the orbit is given there. The electrons of the atom revolve around the nucleus in . Tingnan ang higit paAfter the electron configuration, the last shell of the lithium atom has an electron. In this case, the valence electrons of lithiumare one, and also valency is 1. The elements . Tingnan ang higit pa
67K views 10 years ago. A step-by-step description of how to write the electron configuration for Lithium (Li). In order to write the Li electron configuration we first need to know the.
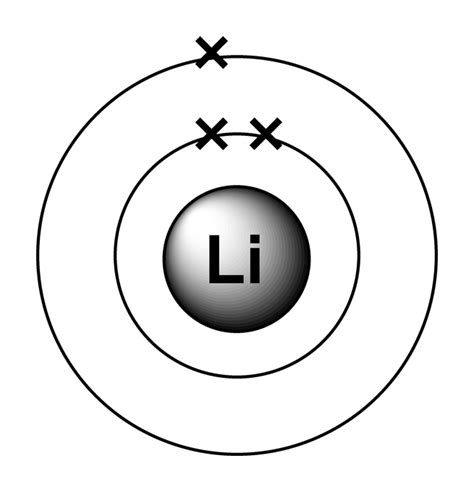
Because lithium’s final electron goes into the 2 s subshell, we write the electron configuration of a lithium atom as 1s22s1. The shell diagram .
In this video we will write the electron configuration for Li+, the Lithium ion. We’ll also look at why Lithium forms a 1+ ion and how the electron configura.
How to Write the Electron Configuration for Lithium. Lithium is the third element with a total of 3 electrons. In writing the electron configuration for lithium the first two .electron arrangement of lithium Lithium Electronic Configuration Its electron configuration will be "Li: " 1s^2 color(red)(2)s^1 Now, the lithium cation, "Li"^(+), is formed when lithium loses the electron located on its outermost shell -> its valence electron. This . Two of the lithium electrons can fit into the 1s subshell, but the third electron must go into the second shell and the lower energy orbital, which is the 2s .
Electron configurationThe arrangements of electrons above the last (closed shell) noble gas. Melting pointThe temperature at which the solid–liquid phase change occurs. . Descriptive Chemistry. Elements Organized by Block. s-Block Elements. Group 1: Hydrogen and the Alkali Metals. Chemistry of Lithium (Z=3) Expand/collapse .Lithium Electronic Configuration The electron configuration of Lithium is: 1s² 2s¹. In this article, we will study how electrons are arranged in different shells and subshells in a Lithium atom. Table of .
The shell closest to the nucleus (first shell) has 2 dots representing the 2 electrons in 1s, while the outermost shell ( 2s) has 1 electron. Figure 2.7.1 2.7. 1: Shell diagram of lithium (Li) atom. The next largest atom, beryllium, has 4 electrons, so its electron configuration is 1 s2 2 s2.
The shell closest to the nucleus (first shell) has 2 dots representing the 2 electrons in 1s, while the outermost shell ( 2s) has 1 electron. Figure 2.7.1 2.7. 1: Shell diagram of lithium (Li) atom. The next largest atom, beryllium, has 4 electrons, so its electron configuration is 1 s2 2 s2.

The arrangement of electrons in the orbitals of an atom is called the electron configuration of the atom. We describe an electron configuration with a symbol that contains three pieces of information ( Figure \(\PageIndex{2}\)): . The first two electrons in lithium fill the 1s orbital and have the same sets of four quantum numbers . Electron configurations describe where electrons are located around the nucleus of an atom. For example, the electron configuration of lithium, 1s²2s¹, tells us that lithium has two .For instance, lithium (Li ) has three electrons: two fill the 1 s orbital, and the third is placed in the 2 s orbital, giving an electron configuration of 1 s 2 2 s 1 . Neon ( Ne ), on the other hand, has a total of ten electrons: two are in its innermost 1 s orbital and eight fill the second shell—two each in the .Lithium is a soft, silvery-white, metal that heads group 1, the alkali metals group, of the periodic table of the elements. It reacts vigorously with water. Storing it is a problem. It cannot be kept under oil, as sodium can, because it is less dense and floats. So it is stored by being coated with petroleum jelly.
It is the arrangement of electrons into shells that has the most effect on chemical properties, so we will focus on mainly on shells here. We use numbers to indicate which shell an electron is in. The first shell, closest to the nucleus and with the lowest-energy electrons, is shell 1. . Lithium has 3 electrons --- 2 in the first shell, and 1 .2.5: Arrangement of Electron (Shell Model) An electron shell is the outside part of an atom around the atomic nucleus. It is a group of atomic orbitals with the same value of the principal quantum number n n. Electron shells have one or more electron subshells, or sublevels. The name for electron shells comes from the Bohr model, in which .The arrangement of electrons in the orbitals of an atom is called the electron configuration of the atom. We describe an electron configuration with a symbol that contains three pieces of information ( Figure \(\PageIndex{2}\)): . The first two electrons in lithium fill the 1s orbital and have the same sets of four quantum numbers as the two .
1s2. Lithium (Z = 3) It has three electrons. Although two can go into the 1s orbital, the third one must be placed in the 2s. 1s22s1. Beryllium (Z = 4) It has four electrons. The fourth can also go in 2s (but remember about the opposing spins) 1s22s2. Boron (Z = 5) It has five electrons. The fifth one must go in 2p.In several cases, the ground state electron configurations are different from those predicted by Figure 6.8.1 6.8. 1. Some of these anomalies occur as the 3 d orbitals are filled. For example, the observed ground state electron configuration of chromium is [Ar]4 s1 3 d5 rather than the predicted [Ar]4 s2 3 d4.
Electron atomic and molecular orbitals A Bohr diagram of lithium. In atomic physics and quantum chemistry, the electron configuration is the distribution of electrons of an atom or molecule (or other physical .electron arrangement of lithium Figure 3.7.1 3.7. 1: Shell diagram of lithium (Li) atom. The next largest atom, beryllium, has 4 electrons, so its electron configuration is 1 s2 2 s2. Now that the 2 s subshell is filled, electrons in larger atoms start filling the 2 p subshell. Thus, the electron configurations for the next six atoms are as follows:
The arrangement of electrons in the orbitals of an atom is called the electron configuration of the atom. We describe an electron configuration with a symbol that contains three pieces of information ( Figure \(\PageIndex{2}\)): . The first two electrons in lithium fill the 1s orbital and have the same sets of four quantum numbers . Aluminum has 13 electrons so it will have the electron arrangement (2, 8, 3) which represents two electrons in the n = 1 n = 1 energy level, eight electrons in the n = 2 n = 2 level, and three electrons in the n = 3 n = 3 level. Aluminum has three valence electrons (indicated by the three electrons in the n = 3 n = 3 level). Example 2.4.4 2.4. 4. In this video we will write the electron configuration for Li+, the Lithium ion. We’ll also look at why Lithium forms a 1+ ion and how the electron configura.Lithium is the third element with a total of 3 electrons. In writing the electron configuration for lithium the first two electrons will go in the 1s orbital. Since 1s can only hold two electrons the remaining electron for Li goes in the 2s orbital. Therefore the Li electron configuration will be 1s 2 2s 1. Lithium is an alkali metal with the atomic number = 3 and an atomic mass of 6.941 g/mol. This means that lithium has 3 protons, 3 electrons and 4 neutrons (6.941 - 3 = ~4). Being an alkali metal, lithium is a soft, flammable, and highly reactive metal that tends to form hydroxides. It also has a pretty low density and under standard conditions .
Hangzhou Sunny Biopharma R&D Co., Ltd. Hangzhou Sunny Biopharma R&D Co., Ltd., located in Xihu District, Hangzhou city, is a wholly owned subsidiary of Sunny Biotech Hangzhou Co., Ltd. Taking advantage of the location and talent advantages of China eastern coastal areas, Sunny Biopharma attracted a large number of international and .
electron arrangement of lithium|Lithium Electronic Configuration